Motivation
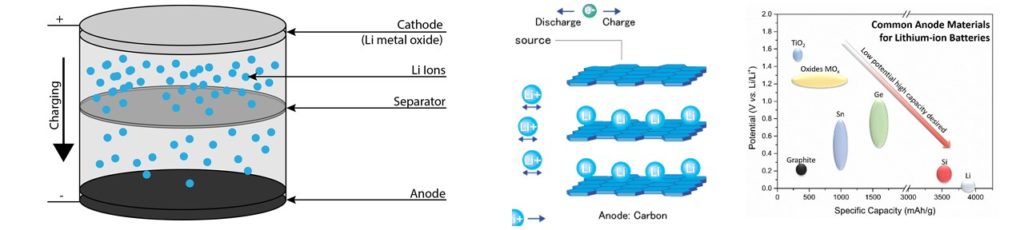
- Lithium-Ion Batteries operate on a “rocking chair” concept (Scrosati1992)
- Lithium atoms from the solid metallic cathode moving through the electrolyte, separator, and inserted into the anode material during charging
- In order to meet the demands of high-performance applications, the materials used in all parts of the cell must work together over repeated cycling
- Anodes made from Carbon (372 mAh/g capacity), silicon (4212 mAh/g) , or mixtures thereof
Proposed Solution
- A composite material with the stability of carbon and the enhanced capacity of silicon
- Combining: oHighly conductive graphitic carbon, oStabile and flexible starch, and oHigh capacity silicon
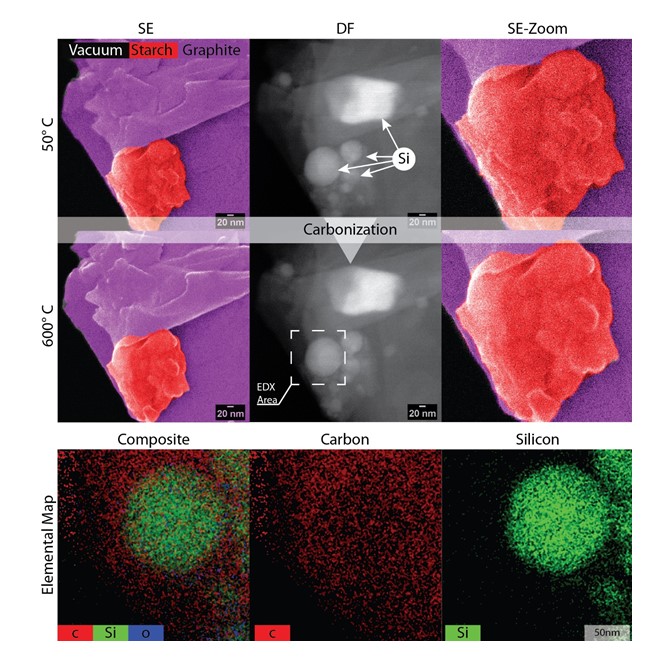
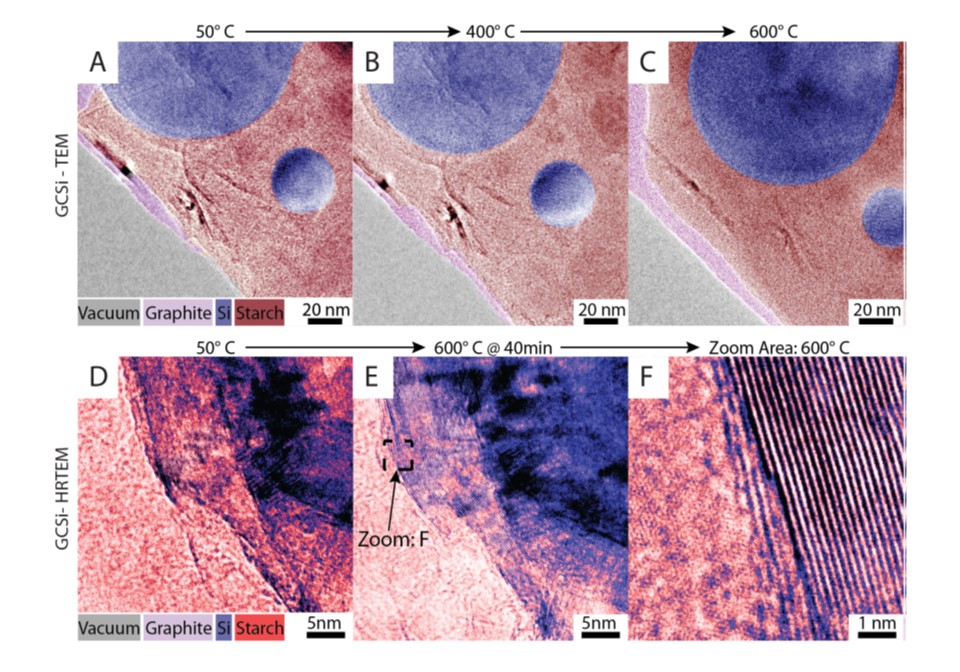
Mixing and Carbonization of GCSI
- The area with all three ingredients
- Sample carbonized up to 6000C
- PAH sheet structure observed
- The gravimetric capacity of GCSi: first discharge cycle 1126 mAh/g; first charge is found to be 937 mAh/g; capacity settles to 500 mAh/g after 100 cycles